Nucleation of Hard Sphere Crystals
Nathan Duff
CHE210D Spring 2009 Final Project
Summary
Hard spheres provide a model of colloid suspensions. Nucleation of hard spheres from a metastable liquid to a crystal was studied. The free energy, ΔG(n) of solid clusters was found to decrease with increasing pressure. Finite size effects for the studied system size (500 spheres) allow for spontaneous crystallization which does not occur in the larger system (3375 spheres) studied by Auer and Frenkel.1 The results suggest a decreased barrier to nucleation due to finite size effects.
Background
Nucleation of colloidal suspensions occurs on experimentally accessible time and length scales2, unlike atomistic systems. Hard spheres provide a model for colloidal suspensions,3-5 which can be compared to experimental results.
Simulation methods
Nucleation of hard spheres was studied in the isobaric-isothermal ensemble (NPT). Pressures were chosen from the work of Auer and Frenkel1 to correspond to packing fractions of 0.5207 to 0.5343 which are within the metastable liquid regime for hard spheres. A system size of 500 was chosen to study the early stages of nucleation.
Monte Carlo simulations were performed to explore the system. Single particle displacements were attempted with a probability of 0.998, and volume change moves were attempted with a probability of 0.002. Nucleation of hard sphere was studied along the reaction coordinate of solid cluster size, n. A sphere was determined to be solid like using Steinhardt local bond order parameter,6 q6 as used by Frenkel and Auer1 for hard spheres. Solid like spheres were than considered to be in the same cluster if the distance between the spheres was less than 1.5 sphere diameters. Free energy of solid cluster sizes between 1 and 10 was found by restricting the system using hard wall constraints.
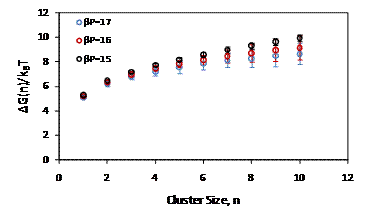
Figure 1: Gibbs free energy as a function of solid cluster size, n.
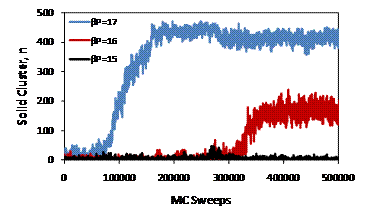
Figure 2: Typical unbiased Monte Carlo trajectories for a system size of N=500. Spontaneous crystallization occurs for βP = 16 and 17.
Results and interpretation
Free energy as a function of cluster size is plotted in Figure 1. The free energy decreases slightly with increasing pressure, although the decrease is often negated by statistical uncertainty. The relative small system size used in this study reduces the barrier to nucleation of solid like spheres. Figure 2 shows typical unbiased Monte Carlo trajectories for pressures of 15, 16, and 17. The existence of spontaneous crystallization trajectories for pressures of 16 and 17 suggest a reduced barrier for nucleation relative to the system size of 3375 used by Auer and Frenkel1 where spontaneous crystallization did not occur.
Calculated free energy barriers to nucleation are generally desired to be independent of system size. As finite size effects resulted in spontaneous crystallization, a larger system size must be used to effectively study nucleation for this system. The calculation of a free energy curve for cluster formation, from a liquid state to a post critical state from which an unconstrained system would crystallize requires important sampling such as umbrella sampling or flat histogram methods.
Movie
Spontaneous crystallization of a system of 500 hard sphere for βP=17 due to finite size effects. Solid like (crystalline) spheres are colored red while liquid like (disordered) spheres are colored grey.
Source code
References
(1) Auer, S.; Frenkel, D. The Journal of Chemical Physics 2004, 120, 3015-3029.
(2) Gasser, U.; Weeks, E. R.; Schofield, A.; Pusey, P. N.; Weitz, D. A. Science 2001, 292, 258-262.
(3) Pusey, P. N.; Vanmegen, W. Nature 1986, 320, 340-342.
(4) Rutgers, M. A.; Dunsmuir, J. H.; Xue, J. Z.; Russel, W. B.; Chaikin, P. M. Physical Review B 1996, 53, 5043-5043.
(5) van Blaaderen, A.; Wiltzius, P. Science 1995, 270, 1177-1179.
(6) Steinhardt, P. J.; Nelson, D. R.; Ronchetti, M. Physical Review B 1983, 28, 784-805.