Capacitor Charging Dynamics of Dilute Soft-Sphere Ions in an Implicit Solvent
Brian Giera
CHE210D Spring 2009 Final Project
Summary
†A dilute system of equally sized cations and anions suspended in an implicit solvent between two capacitor plates was simulated. Concentration profiles were generated as a function of time and the behavior of double layer formation was observed after the plates were charged. This model can be used to investigate double layer formation, which is a critical mechanism of supercapacitor charging.
Background
This model simulates capacitor plates separated by a dilute electrolyte that, when charged, attract oppositely charged counter-ions to its surface. This electrostatic attraction is balanced by the ionsí tendency to travel down concentration gradients (away from the surface) forming a non-conductive region called the electric double layer (EDL).† Supercapacitors also exploit charge separation across EDLs that form over extremely high surface area electrodes. This simplified model investigates transient EDL formation, which is relevant to supercapacitor charging.
Simulation methods
All the particles in the system interact via the following soft-sphere repulsion with a screened Coulomb potential that accounts for the implicit solvent:
![]() ;
;
† where 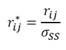 ,
,![]() ,
,![]() , and
, and ![]() to give
to give
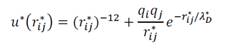 ;
;
The dimensionless
temperature and volume equal ![]() †and
†and ![]() , respectively.
, respectively.
rij is the position vector between particle i and j, qi and qj are the coefficients of charge on the particles, σSS is the soft sphere diameter (set equal to 1 for all particles), λD is the Debye screening length (set equal to 5σSS), єCoul is the Coulombic energy scale that is equal to the square of the fundamental charge, e0, divided by the product of the dielectric permittivity and soft-sphere diameter, 4πє0єσss. The electrolyte was made of fifty cations and fifty anions with an overall bulk density of 0.1. Each capacitor plate is comprised of one hundred particles that only interact with the ions; there are no intra-plate interactions and the plates do not impose a force upon each other. Periodic conditions were imposed only in the x- and y-directions.
††††††††††††††† Seven molecular dynamics simulations were performed for both T*=1 and T*=3 over 250000 time steps with dt=0.001.† A Berendsen thermostat with a time constant equal to 0.01 (=dt/0.01) was used to maintain the temperature. The particles were initially placed in a cubic lattice and given random velocities. After 8 dimensionless time units, the walls were charged and the formation of the double layer was observed.
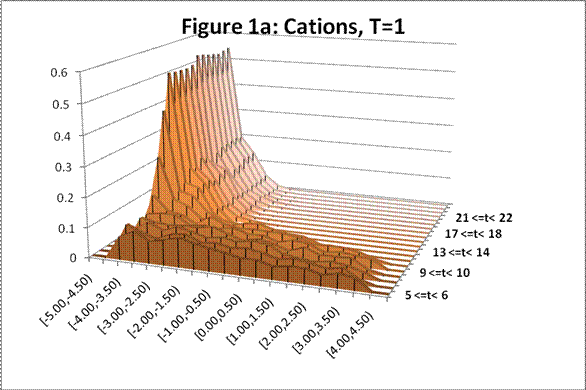
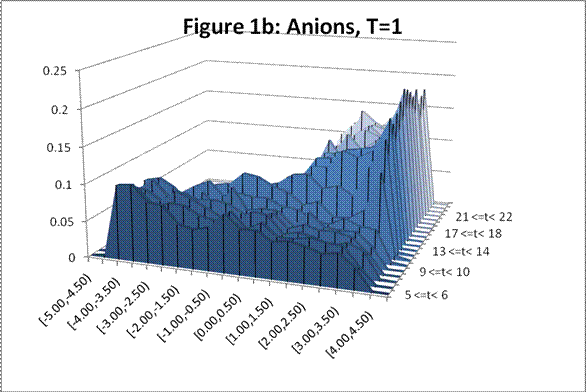
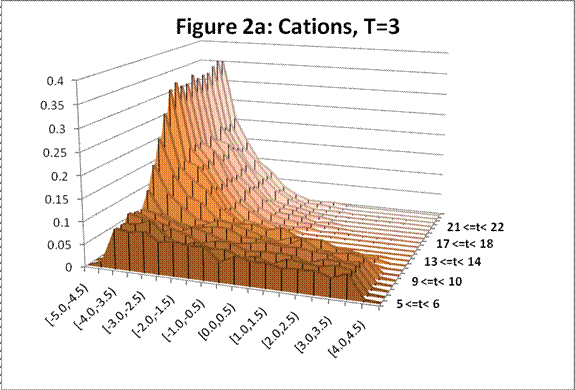
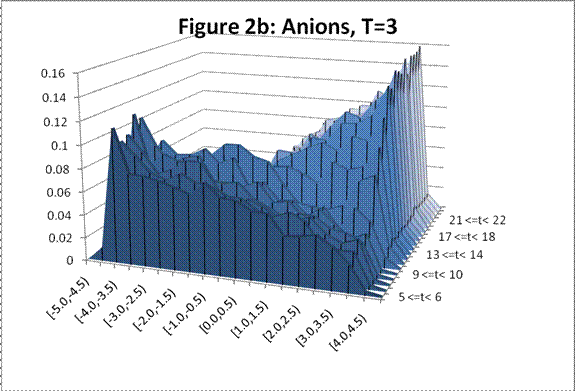
The following charts show the change in density profile for the ions through time at the simulated temperatures indicated:
z-height z-height
![]()
![]()
z-height z-height
![]()
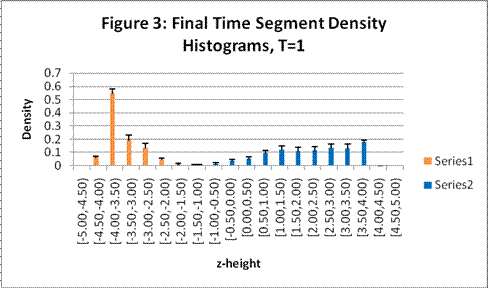
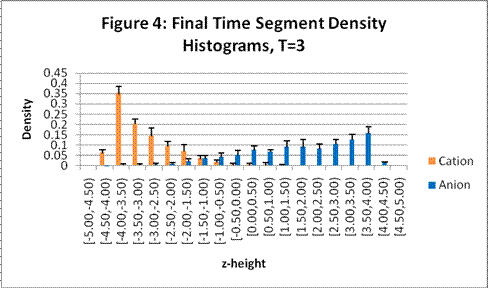
These charts show the density profile for the last time segment and include error bars:
Results and interpretation
This system exhibits the formation of the double layer during capacitor charging. The final concentration profiles also agree with those of the Guoy-Chapman theory in the Debye-Hückel limit, which demands low ionic strengths of monovalent ions [1].† For both observed temperatures, the ions accumulate on the surfaces of the oppositely charged plates over time as shown in Figures 1 and 2. Higher temperatures resulted in lower ionic concentrations at the capacitor plates due to greater thermal motion. The ion profiles in Figures 3 and 4 generated for the final time segment exhibit exponential density decay away from the surface. Lastly, the two cation profiles have higher maximum densities than those of the anion profiles. This can be explained by Figures 1 and 2, which demonstrate relatively higher initial anion densities at the bottom plate. The nonsymmetrical density profiles was due to the initial placement of the cubic lattice, which was closer to the bottom plate. The ions did not evenly distribute themselves in the time before the charge was applied. Symmetric density profiles would be expected for systems in which the electrolytes were randomly distributed when the charge was applied.
This model can be further improved by use of an Ewald summation to compute the electrostatic potentials. This model will also be updated to include an explicit solvent. The walls do not interact with each other, but evaluating their attractive force during double layer formation may be relevant to surface force apparatus (SFA) applications. Furthermore, additional information regarding the effects of different length scales, particle densities, temperatures, applied charge, and time scales are to be investigated. The systemís behavior using differently sized particles would also be useful in investigating steric effects.
Movie
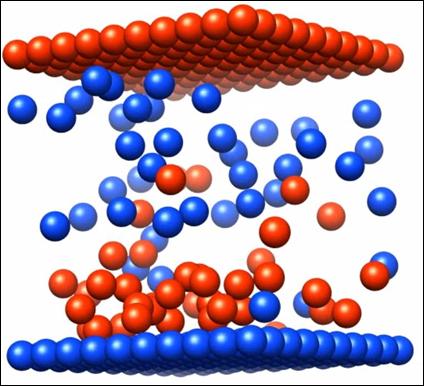
Eighty oppositely charged (and oppositely colored) particles are placed in a cubic lattice and allowed to randomly diffuse in an implicit solvent for eight dimensionless time units (DTUs). The initially grey capacitor plates change color as soon as a charge is applied. The electric double layer can be seen to form over the remaining seventeen DTUs as ions are attracted to the charged surface.
Source code
References
[1] Russel, W.B., Saville, D.A., & Schrowalter, W.R., 1984 Colloidal Dispersions. Cambridge University Press.