Click on thumbnails to enlarge figures and display Octave/Matlab code and data.
You will also need a few files (mostly for plotting) that are included
only in the tar.gz or zip files linked below, so if you want to
run them, the best thing to do is download the complete collection
of M-files! Having the code linked here is still useful if you want
to take a quick look at how something is done.
Complete collections of the M-files for both Octave and Matlab
in zip file formats are available for download from the
following links:

|
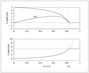
Figure 4.3 (page 113):
First-order, irreversible kinetics in a batch reactor.
|
| |

|
Figure 4.4 (page 113):
First-order, irreversible kinetics in a batch reactor, log scale.
|
| |
|
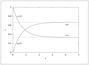
Figure 4.5 (page 116):
First-order, reversible kinetics in a batch reactor, k_1=1, k_{-1}=0.5, c_{A0}=1, c_{B0}=0.
|
| |

|
Figure 4.6 (page 118):
Second-order and first-order kinetics in a batch reactor; for second-order, kc_{A0}=1, and for first order, k=1, so the rates are equal initially.
|
| |

|
Figure 4.7 (page 120):
Reaction rate versus concentration for nth-order kinetics, r=kc_A^n, n\geq 0, k=1 for all orders.
|
| |

|
Figure 4.8 (page 122):
Batch reactor with nth-order kinetics, r=kc_A^n, k_0=kc_{A0}^{n-1}=1, n\geq 1.
|
| |

|
Figure 4.9 (page 122):
Batch reactor with nth-order kinetics, r=kc_A^n, k_0=kc_{A0}^{n-1}=1, n<1.
|
| |

|
Figure 4.10 (page 123):
Reaction rate versus concentration for nth-order kinetics, r=kc_A^n, n\leq 0, k=1 for all orders.
|
| |

|
Figure 4.11 (page 125):
Two first-order reactions in series in a batch reactor.
|
| |

|
Figure 4.12 (page 126):
Two first-order reactions in parallel in a batch reactor.
|
| |
|
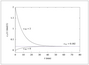
Figure 4.14 (page 130):
Reaching steady state in a CSTR.
|
| |
|
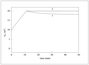
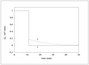
Figure 4.15 (page 142):
Semi-batch reactor volume for different monomer addition policies.
|
| |
|
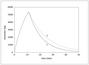
Figure 4.16 (page 142):
Semi-batch reactor feed flowrate for different monomer addition policies.
|
| |
|
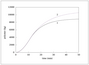
Figure 4.17 (page 143):
Semi-batch reactor monomer content for different monomer addition policies.
|
| |
|
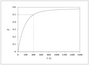
Figure 4.18 (page 143):
Semi-batch reactor polymer content for different monomer addition policies.
|
| |
|
Figure 4.20 (page 152):
Benzene conversion versus reactor volume.
|
| |

|
Figure 4.21 (page 152):
Component mole fractions versus reactor volume.
|
| |

|
Figure 4.22 (page 157):
Molar flowrate of ethane, ethylene and NO versus reactor volume for ethane pyrolysis example.
|
| |

|
Figure 4.23 (page 157):
Molar flowrate of ethane versus reactor volume for inlet temperatures of 1000, 1050 and 1100~K.
|
| |
|
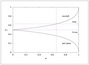
Figure 4.27 (page 162):
Overall and per-pass conversion of A as a function of fractional recycle, \alpha .
|
| |

|
Figure 4.29 (page 166):
Stochastic simulation of the first-order reactions A-> B-> C starting with 100 A~molecules.
|
| |

|
Figure 4.30 (page 167):
Stochastic simulation of the first-order reactions A-> B-> C starting with 1000 A~molecules.
|
| |

|
Figure 4.31 (page 167):
Stochastic simulation of the first-order reactions A-> B-> C starting with 4000 A~molecules.
|
| |

|
Figure 4.32 (page 169):
Species cccDNA versus time for hepatitis B virus model; deterministic and average stochastic models.
|
| |

|
Figure 4.33 (page 169):
Species rcDNA versus time for hepatitis B virus model; deterministic and average stochastic models.
|
| |

|
Figure 4.34 (page 169):
Envelope versus time for hepatitis B virus model; deterministic and average stochastic models.
|
| |

|
Figure 4.35 (page 170):
Species cccDNA versus time for hepatitis B virus model; two representative stochastic trajectories.
|
| |

|
Figure 4.37 (page 182):
Deterministic simulation of reaction A + B <-> C compared to stochastic simulation.
|
| |

|
Figure 5.1 (page 196):
Morse potential for H_2 and HF.
|
| |

|
Figure 5.2 (page 197):
Potential-energy surface for the F, H_2 reaction.
|
| |
|
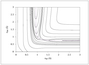
Figure 5.3 (page 198):
Contour representation of the potential-energy surface.
|
| |

|
Figure 5.4 (page 199):
Reaction-coordinate diagram.
|
| |

|
Figure 5.5 (page 208):
Comparison of measured and calculated rate constant versus temperature for trioxane decomposition.
|
| |

|
Figure 5.6 (page 210):
Full model solution for k_1=1, k_{-1}=0.5, k_2=k_{-2}=1.
|
| |

|
Figure 5.7 (page 210):
Full model solution for k_1=1, k_{-1}=0.5, k_2=k_{-2}=10.
|
| |

|
Figure 5.8 (page 211):
Concentrations of B and C versus time for full model with increasing k_2 with K_2=k_2/k_{-2}=1.
|
| |

|
Figure 5.9 (page 214):
Comparison of equilibrium assumption to full model for k_1=1, k_{-1}=0.5, k_2=k_{-2}=10.
|
| |

|
Figure 5.10 (page 221):
Normalized concentration of C versus dimensionless time for the series reaction A -> B -> C.
|
| |

|
Figure 5.11 (page 224):
Fractional error in the QSSA concentration of C for the series reaction A -> B -> C.
|
| |

|
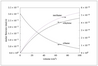
Figure 5.12 (page 225):
Fractional error in the QSSA concentration of C versus dimensionless time for the series-parallel reaction, A<-> B-> C.
|
| |
|
Figure 5.13 (page 234):
Molar flowrates of C_2H_6, C_2H_4 and CH_4 corresponding to the exact solution.
|
| |

|
Figure 5.14 (page 236):
Fractional error in the QSSA molar flowrate of C_2H_4 versus reactor volume.
|
| |

|
Figure 5.15 (page 237):
Comparison of the molar flowrates of C_2H_6 and C_2H_4 for the exact solution (solid lines) and the simplified kinetic scheme (dashed lines).
|
| |

|
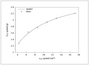
Figure 5.18 (page 243):
Fractional coverage versus adsorbate concentration for different values of the adsorption constant, K.
|
| |
|
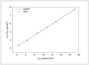
Figure 5.19 (page 245):
Langmuir isotherm for CO uptake on Ru.
|
| |
|
Figure 5.20 (page 245):
Linear form of Langmuir isotherm for CO uptake on Ru.
|
| |

|
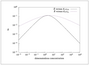
Figure 5.23 (page 252):
Dimensionless CO_2 production rate versus dimensionless gas-phase CO and O_2 concentrations.
|
| |
|
Figure 5.24 (page 253):
Dimensionless CO_2 production rate versus a single dimensionless gas-phase concentration.
|
| |

|
Figure 5.25 (page 263):
Adsorbed oxygen concentration versus gas-phase oxygen concentration.
|
| |
|
|
|

|
Figure 6.4 (page 288):
Conversion of A versus reactor temperature.
|
| |

|
Figure 6.5 (page 291):
Steady-state conversion versus residence time for different values of the heat of reaction.
|
| |

|
Figure 6.6 (page 291):
Steady-state temperature versus residence time for different values of the heat of reaction.
|
| |

|
Figure 6.7 (page 292):
Steady-state conversion versus residence time for \Delta H_R = -3 x10^5~kJ/kmol; ignition and extinction points.
|
| |

|
Figure 6.8 (page 292):
Steady-state temperature versus residence time for \Delta H_R = -3 x10^5~kJ/kmol; ignition and extinction points.
|
| |

|
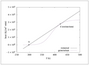
Figure 6.9 (page 294):
Steady-state temperature versus residence time for \Delta H_R = -3 x10^5~kJ/kmol.
|
| |
|
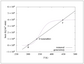
Figure 6.10 (page 296):
Rates of heat generation and removal for \tau =1.79~min.
|
| |
|
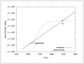
Figure 6.11 (page 297):
Rates of heat generation and removal for \tau =15~min.
|
| |
|
Figure 6.12 (page 297):
Rates of heat generation and removal for \tau =30.9~min.
|
| |

|
Figure 6.13 (page 301):
Eigenvalues of Jacobian matrix vs. reactor temperature.
|
| |

|
Figure 6.14 (page 301):
Eigenvalues of Jacobian matrix vs. reactor conversion.
|
| |

|
Figure 6.18 (page 304):
Steady-state conversion versus residence time.
|
| |

|
Figure 6.19 (page 304):
Steady-state temperature versus residence time.
|
| |

|
Figure 6.20 (page 305):
Steady-state conversion vs. residence time---log scale.
|
| |

|
Figure 6.21 (page 305):
Steady-state temperature vs. residence time---log scale.
|
| |

|
Figure 6.22 (page 307):
Eigenvalues of the Jacobian matrix versus reactor conversion in the region of steady-state multiplicity.
|
| |

|
Figure 6.23 (page 307):
Eigenvalues of the Jacobian matrix versus reactor conversion in the region of steady-state multiplicity.
|
| |

|
Figure 6.24 (page 308):
Real and imaginary parts of the eigenvalues of the Jacobian matrix near points C--F.
|
| |

|
Figure 6.25 (page 309):
Conversion and temperature vs. time for \tau =35~min.
|
| |

|
Figure 6.26 (page 310):
Phase portrait of conversion versus temperature for feed initial condition; \tau =35~min.
|
| |

|
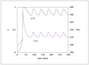
Figure 6.27 (page 310):
Phase portrait of conversion versus temperature for several initial conditions; \tau =35~min.
|
| |
|
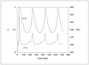
Figure 6.28 (page 311):
Conversion and temperature vs. time for \tau =30~min.
|
| |
|
Figure 6.29 (page 311):
Conversion and temperature vs. time for \tau =72.3~min.
|
| |
|
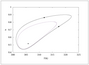
Figure 6.30 (page 312):
Phase portrait of conversion versus temperature at showing stable and unstable limit cycles.
|
| |

|
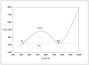
Figure 6.35 (page 323):
Molar flow of o-xylene versus reactor length for different feed temperatures.
|
| |

|
Figure 6.36 (page 323):
Reactor temperature versus length for different feed temperatures.
|
| |
|
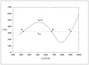
Figure 6.38 (page 328):
Coolant temperature at reactor outlet versus temperature at reactor inlet; three steady-state solutions.
|
| |
|
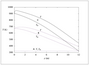
Figure 6.39 (page 329):
Reactor and coolant temperature profiles versus reactor length.
|
| |
|
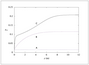
Figure 6.40 (page 329):
Ammonia mole fraction versus reactor length.
|
| |
|
Figure 6.41 (page 346):
Coolant temperature at reactor outlet versus temperature at reactor inlet.
|
| |
|
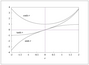
Figure 7.3 (page 365):
Hyperbolic trigonometric functions sinh, cosh, tanh.
|
| |

|
Figure 7.4 (page 365):
Dimensionless concentration versus dimensionless radial position for different values of the Thiele modulus.
|
| |

|
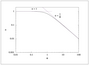
Figure 7.5 (page 368):
Effectiveness factor versus Thiele modulus for a first-order reaction in a sphere.
|
| |
|
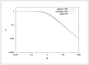
Figure 7.6 (page 368):
Effectiveness factor versus Thiele modulus for a first-order reaction in a sphere (log-log scale).
|
| |
|
Figure 7.8 (page 372):
Effectiveness factor versus Thiele modulus for the sphere, cylinder and slab.
|
| |

|
Figure 7.9 (page 374):
Effectiveness factor versus Thiele modulus in a spherical pellet; reaction orders greater than unity.
|
| |

|
Figure 7.10 (page 374):
Effectiveness factor versus Thiele modulus in a spherical pellet; reaction orders less than unity.
|
| |

|
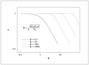
Figure 7.11 (page 375):
Dimensionless concentration versus radius for zero-order reaction in a spherical pellet.
|
| |
|
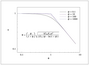
Figure 7.13 (page 380):
Effectiveness factor versus an inappropriate Thiele modulus in a slab; Hougen-Watson kinetics.
|
| |
|
Figure 7.14 (page 380):
Effectiveness factor versus appropriate Thiele modulus in a slab; Hougen-Watson kinetics.
|
| |

|
Figure 7.16 (page 383):
Dimensionless concentration versus radius for different values of the Biot number; first-order reaction in a spherical pellet with \Phi =1.
|
| |

|
Figure 7.17 (page 384):
Effectiveness factor versus Thiele modulus for different values of the Biot number; first-order reaction in a spherical pellet.
|
| |

|
Figure 7.18 (page 385):
Asymptotic behavior of the effectiveness factor versus Thiele modulus; first-order reaction in spherical pellet.
|
| |

|
Figure 7.19 (page 390):
Effectiveness factor versus normalized Thiele modulus for a first-order reaction in nonisothermal spherical pellet.
|
| |

|
Figure 7.20 (page 392):
Dimensionless concentration versus radius for the nonisothermal spherical pellet: lower (A), unstable middle (B), and upper (C) steady states.
|
| |

|
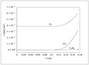
Figure 7.21 (page 392):
Dimensionless temperature versus radius for the nonisothermal spherical pellet: lower (A), unstable middle (B), and upper (C) steady states.
|
| |
|
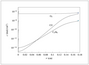
Figure 7.22 (page 395):
Concentration profiles of reactants; fluid concentration of O_2 (x), CO (+), C_3H_6 (*).
|
| |
|
Figure 7.23 (page 395):
Concentration profiles of reactants (log scale); fluid concentration of O_2 (x), CO (+), C_3H_6 (*).
|
| |

|
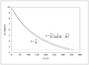
Figure 7.24 (page 397):
Concentration profiles of products.
|
| |
|
Figure 7.26 (page 404):
Molar flow of A versus reactor volume for second-order, isothermal reaction in a fixed-bed reactor.
|
| |

|
Figure 7.27 (page 407):
Molar concentrations versus reactor volume.
|
| |

|
Figure 7.28 (page 407):
Dimensionless equilibrium constant and Thiele modulus versus reactor volume.
|
| |

|
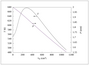
Figure 7.29 (page 410):
Fluid molar concentrations versus reactor volume.
|
| |
|
Figure 7.30 (page 410):
Fluid temperature and pressure versus reactor volume.
|
| |

|
Figure 7.32 (page 411):
Pellet CO profiles at several reactor positions.
|
| |

|
Figure 7.34 (page 424):
Effectiveness factor versus Thiele modulus for different values of the Biot number; second-order reaction in a cylindrical pellet.
|
| |

|
Figure 8.5 (page 437):
CSTR residence-time distribution.
|
| |

|
Figure 8.8 (page 441):
RTD p(\theta ) versus \theta for n CSTRs in series, \tau =2.
|
| |

|
Figure 8.9 (page 442):
P(\theta ) versus \theta for n CSTRs in series, \tau =2.
|
| |

|
Figure 8.10 (page 445):
P(\theta ) versus \theta for plug flow with dispersion number D, \tau =2.
|
| |

|
Figure 8.11 (page 445):
Residence-time distribution p(\theta ) versus \theta for plug flow with dispersion number D, \tau =2.
|
| |

|
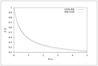
Figure 8.12 (page 447):
Start-up of the tubular reactor; c_A(t,z) versus z for various times, 0\leq t\leq 2.5~min, \Delta t=0.25~min.
|
| |
|
Figure 8.14 (page 450):
Comparison of the effluent concentrations for the two cases shown in Figure~\ref {fig:pc_cp_schem}.
|
| |

|
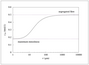
Figure 8.19 (page 459):
Dimensionless effluent concentration versus dimensionless rate constant for second-order reaction.
|
| |
|
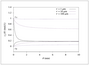
Figure 8.22 (page 463):
Total concentration of A in the reactor effluent versus particle size.
|
| |
|
Figure 8.23 (page 463):
Particle concentrations of A and B versus particle age for three different-sized particles.
|
| |

|
Figure 8.29 (page 470):
Reaction rate versus concentration of limiting reactant; rate expression is neither convex nor concave.
|
| |
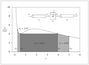
|
Figure 8.30 (page 471):
Inverse of reaction rate versus concentration; optimal sequence to achieve 95% conversion is PFR--CSTR--PFR.
|
| |

|
Figure 8.31 (page 472):
RTD for the optimal reactor configuration.
|
| |

|
Figure 8.33 (page 479):
Conversion of reactant for single, ideal CSTR, and as a function of internal flowrate in a 2-CSTR mixing model.
|
| |

|
Figure 8.34 (page 479):
Yield of desired product C for single, ideal CSTR, and as a function of internal flowrate, \rho =Q_r/Q_2, in a 2-CSTR mixing model.
|
| |

|
Figure 8.35 (page 480):
Step response for single, ideal CSTR, and 2-CSTR mixing model with \rho =0,1.
|
| |

|
Figure 8.36 (page 483):
Conversion of reactant A versus reactor length for different dispersion numbers.
|
| |

|
Figure 8.37 (page 483):
Yield of desired product B versus reactor length for different dispersion numbers.
|
| |

|
Figure 8.39 (page 487):
Tracer concentrations in the feed and effluent streams versus time.
|
| |

|
Figure 8.41 (page 491):
Effluent concentration versus time after unit step change in the first reactor.
|
| |

|
Figure 9.4 (page 511):
Univariate normal with zero mean and unit variance.
|
| |

|
Figure 9.5 (page 512):
Multivariate normal for n_p=2.
|
| |

|
Figure 9.7 (page 516):
Measured rate constant at several temperatures.
|
| |

|
Figure 9.8 (page 517):
Transformed data set, \ln k versus 1/T.
|
| |

|
Figure 9.9 (page 517):
Several replicate data sets, \ln k versus 1/T.
|
| |

|
Figure 9.10 (page 518):
Distribution of estimated parameters.
|
| |

|
Figure 9.11 (page 519):
Reducing parameter correlation by centering the data.
|
| |
|
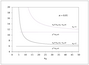
Figure 9.12 (page 521):
Values of \chi ^2 and F versus the number of data points when estimating 2 and 5 parameters.
|
| |
|
Figure 9.13 (page 522):
Parameter estimates with only 10 data points.
|
| |

|
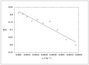
Figure 9.14 (page 523):
Confidence intervals with known (solid line) and unknown (dashed line) error variance.
|
| |
|
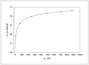
Figure 9.15 (page 526):
Model fit to a single adsorption experiment.
|
| |

|
Figure 9.16 (page 527):
Model fit to all adsorption experiments.
|
| |

|
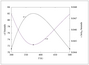
Figure 9.18 (page 530):
Effect of next measurement temperature on parameter confidence intervals.
|
| |
|
Figure 9.19 (page 532):
Uncertainty in activation energy E and rate constant \ln k_m versus next measurement temperature.
|
| |

|
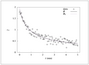
Figure 9.20 (page 533):
Uncertainty in activation energy E and rate constant \ln k_m versus number of replicated experiments.
|
| |
|
Figure 9.21 (page 538):
Experimental measurement and best parameter fit for nth-order kinetic model, r=k c_A^n.
|
| |

|
Figure 9.22 (page 539):
Monte Carlo evaluation of confidence intervals.
|
| |

|
Figure 9.23 (page 542):
Species cccDNA versus time for hepatitis B virus model; initial guess and estimated parameters fit to data.
|
| |

|
Figure 9.24 (page 542):
Species rcDNA versus time for hepatitis B virus model; initial guess and estimated parameters fit to data.
|
| |

|
Figure 9.25 (page 542):
Envelope versus time for hepatitis B virus model; initial guess and estimated parameters fit to data.
|
| |

|
Figure 9.26 (page 546):
Species cccDNA versus time for hepatitis B virus model.
|
| |

|
Figure 9.27 (page 546):
Species rcDNA versus time for hepatitis B virus model.
|
| |

|
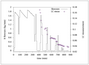
Figure 9.28 (page 546):
Envelope versus time for hepatitis B virus model.
|
| |
|
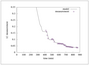
Figure 9.31 (page 551):
Base addition rate and LC measurement versus time.
|
| |
|
Figure 9.32 (page 552):
Comparison of data to model with optimal parameters.
|
| |

|
Figure 9.33 (page 553):
Total amount of species A, C and D versus time.
|
| |

|
Figure 9.34 (page 553):
Total amount of species B versus time.
|
| |

|
Figure 9.35 (page 556):
Predictions of LC measurement for reduced model.
|
| |

|
Figure 9.36 (page 558):
Fit of LC measurement versus time for reduced model; early time measurements have been added.
|
| |
|
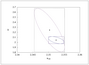
Figure 9.37 (page 558):
Confidence intervals for reduced model without (dashed) and with (solid) redesigned experiment.
|
| |

|
Figure 9.38 (page 562):
Batch-reactor data for Exercise~{\ref {exer:batch_data}}.
|
| |

|
Figure 9.39 (page 563):
Batch-reactor data for Exercise~{\ref {exer:batch_noise}}; 3 runs with different measurement error variance.
|
| |

|
Figure 9.40 (page 566):
Batch-reactor data for Exercise~{\ref {exer:react2rev}}.
|
| |

|
Figure 9.41 (page 566):
A second experiment for Exercise~{\ref {exer:react2rev}}.
|
| |

|
Figure 9.42 (page 568):
Batch-reactor data for Exercise~{\ref {exer:react2}}.
|
| |

|
Figure 9.43 (page 568):
A second experiment for Exercise~{\ref {exer:react2}}.
|
| |

|
Figure A.1 (page 636):
Estimated reaction rates from 2000 production-rate measurements subject to measurement noise.
|
| |

|
Figure A.2 (page 640):
Gibbs energy contours for the pentane reactions as a function of the two reaction extents.
|
| |

|
Figure A.3 (page 650):
Solution to first-order differential equation dc_A/dt=-kc_A, and sensitivities S_1=d c_A/d k and S_2=d c_A/d c_{A0}.
|
| |

|
Figure A.5 (page 654):
Dimensionless concentration versus dimensionless radial position for different numbers of collocation points.
|
| |

|
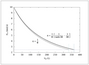
Figure A.6 (page 655):
Relative error in the effectiveness factor versus number of collocation points.
|
| |
|
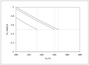
Figure A.7 (page 656):
Molar flow of A versus reactor volume for second-order, isothermal reaction in a fixed-bed reactor; two approximations and exact solution.
|
| |
|
Figure A.8 (page 656):
Magnified view of Figure~{\ref {fig:fb2colloc}}.
|
| |

|
Figure A.9 (page 658):
Measurements of species concentrations in Reactions~{\ref {rxn:ABC}} versus time.
|
| |

|
Figure A.10 (page 658):
Fit of model to measurements using estimated parameters.
|
| |

|
Figure A.11 (page 662):
Dimensionless concentration versus radius for the nonisothermal spherical pellet: lower (A), unstable middle (B), and upper (C) steady states.
|
| |
![[Textbook cover goes here]](front_cover_screenshot.png)